

Chemical reactions are the stuff of life. When we cook food, start a motor, or washing our hands in soap, we depend on chemical reactions. There are many types of reactions, but all of them have one thing in common. They have to be balanced.
What does “balance” mean?
It means that the charges on both sides of the equation must cancel out to be neutral.
An atom is made up of protons, neutrons, and electrons.
Protons have a positive charge (+)
Neutrons have no charge so we can forget about them for simplicity’s sake.
Electrons have a negative charge (-)
An atom looks like this:
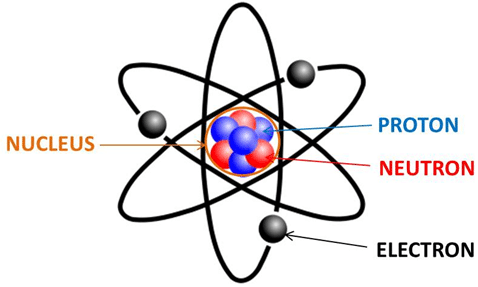
The nucleus, which is made up of protons and neutrons, has a positive charge, due to the protons. The number of orbiting electrons balances the number of protons so that an atom ideally is neither positive nor negative.
But if an atom LOSES an electron, that means the atom becomes positively charged. Look at the picture above. See how it has FOUR protons, FOUR neutrons, and THREE electrons? This breaks down to FOUR positive charges, FOUR neutrals, and THREE negatives. They cancel each other out, leaving ONE positive charge left. So this atom has a charge of +1.
If this atom GAINS an electron, it will have FOUR electrons to match the FOUR protons. Its charge will be ZERO.
If this atom GAINS TWO electrons, it will have FIVE electrons to the FOUR protons. Its charge will be -1.
An atom that gains or loses an electron is called an ION. An atom that LOSES an electron for a positive charge is called an CATION (pronounced cat-ion). An atom that GAINS an electron for a negative charge is called an ANION (pronounced an-ion).
With me so far? Okay, now let’s look at the periodic table.
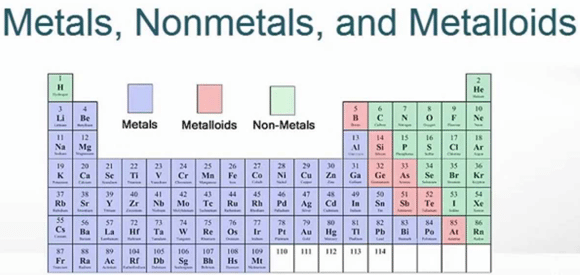
See how it’s color coded? The table is divided into metals and nonmetals (and metalloids, which mean they can be both, but we won’t worry about those). These processes are important to understanding the molar mass of an element.
Metals are purple and to the left (except for Hydrogen at the top). Metals tend to LOSE electrons to create positive charges.
The periodic table gives you a hint: the atoms at the extreme left lose ONE electron for a +1 charge. Li, Na, K, Rb, Cs, and Fr all lose ONE electron in a reaction. Their ions have a +1 charge.
The next column to the left tend to lose TWO electrons in a chemical reaction, for a +2 charge. Be, Mg, Ca, Sr, Ba, and Ra ions all have +2 charges.
We’ll leave the shorter stacks out for now. Those ions’ charges vary.
On the right side are the non-metals. These are gases like helium and nitrogen, liquids like chlorine and iodine, and substances like sulfur. The non-metals are tricky.
That is because many of these elements exist in molecules consisting of 2 atoms. Oxygen gas exists as O2. Nitrogen gas exists as N2. Liquid chlorine is Cl2.
Tip: We do not have to worry about the extreme right. They are ALWAYS balanced. He, Ne, Ar, Kr, Xe, and Rn generally do not react with anything. They are also found in molecules of 2: He2, Xe2, etc. These are called “noble gases” because they do not react.
In a chemical reaction, many of these molecules break up into individual anions. These anions have a negative charge. The column to the left of the noble gases GAINS ONE electron. F, Cl, Br, and I anions have a -1 charge. The next column gains TWO electrons: O, S, and Se anions have a -2 charge. In the next column, Nitrogen (N) has a -3 charge.
There are 2 exceptions. Carbon (C) can either gain or lose electrons. Way over on the left hand side, Hydrogen (H) ALWAYS loses its electrons for a +1 charge. That is why it is placed with Li and other positive ions.
So, let’s recap all this:
Metals: always LOSE electrons for ions with positive charges.
Non-metals: mostly GAIN electrons for anions with negative charges. Many non-metals have a pure state of TWO atoms joined together: H2, F2, S2, etc.
Take a breather—this is where the fun begins!
Let’s put it together with some basic chemical reactions.
Common table salt has a chemical label of NaCl. That means it has one Sodium (Na +1 ) cation and one Chlorine (Cl -1 ) anion.
How do we make salt? We just join the two atoms. Na is in the far left column, which means it LOSES ONE electron. Cl is in the non-metal column that GAINS one electron. So the two are a perfect fit. This is why salt is so common and cheap!

Na +1 + Cl -1 ->NaCl.
Now to make it more complicated.
Remember how Cl only exists in a pure state as two atoms joined together? Cl2. What happens to the other Cl atom? It needs to gain an electron. The easiest solution is to add another Na +1 cation for it to hook up with. So, our formula now reads:
2Na +1 + Cl2 -> 2NaCl
Note the placement of the 2. NaCl is a balanced formula, so we can’t change it to read anything else (NaCl2 would imply that one molecule of salt has two chlorine atoms for one sodium atom and this is not correct. The ratio is 1:1.Na2Cl2 is also incorrect because one molecule has two Na and two Cl, while in reality each molecule only has one). But we have to account for every atom and for every charge. The Na atoms have a +1 charge each. When we break up Cl2, each Cl anion has -1 charge each. Placed together, these positive and negative charges cancel each other out and each atom is accounted for.
**(There are some other things we can add to the equation, such as the presence of heat that is released during the reaction, and that these reactions take place in solutions, but that’s getting ahead of the game).
Think you got it? Let’s try something else: Magnesium Bromide.
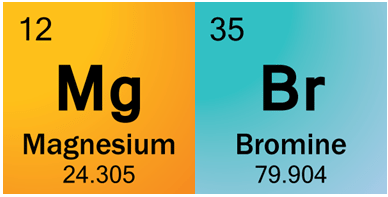
Magnesium cation is in the second to left column, so it has a +2 charge.
Bromine is in the second to right column, so it has a -1 charge.
Technically, you can write:
Mg +2 + 2Br -1 -> MgBr2.
Even though we need 2 Br atoms to cancel the +2 positive charge on Mg, we have to remember that Bromine does not exist as individual atoms in a natural state. Like Chlorine, Bromine’s natural state is two atoms joined together as Br2.
So the right answer is:
How about water? We all know this one: H2O!
O gains 2 electrons while each H loses one electron. We know we need two Hydrogen atoms for each Oxygen atom.
Hydrogen loses atoms, so it has a positive charge. It also exists as an H2 molecule in its natural state, so we’re okay there.
But Oxygen also exists as O2in its natural state. We need to account for the extra O!
Let’s put it together:
Almost there! We need another O on the right to balance the number of Os on the left. We can’t write the final equation as H2O2 because one molecule of water only has one oxygen atom and we would be stuck with an extra -2 charge because of the second O. (It would look like H2O2 -2 YIKES!). We can simply add an extra water molecule altogether:
Now we have TWO Oxygen atoms on each side. But now we have FOUR Hydrogens on the right (TWO molecules of water, each with TWO hydrogens). How can we balance the FOUR on the right and TWO on the left?
Add another Hydrogen molecule:
Now everything matches up: Four Hydrogen and Two Oxygen atoms on each side. This is balanced! After three examples, the process should start getting clearer now. If it doesn’t, you should consider getting some chemistry homework help.
Want something really horrible to try.
Let’s start with a balanced formula: HSO3
Let’s break this down. Hold on to your hat!
We know H has a +1 charge. That means SO3has a -1 charge altogether.
We’ll keep SO3 intact for this equation. It’s pretty hard to break up this molecule (for reasons that are too complex to get into for now. ).
So, what is HSO3 good for, anyway, you ask? It helps soften your water!
Hard water is the presence of Calcium (Ca), or Calcium chloride (CaCl2) which is left behind when water dries. It clogs your drains and leaves deposits on your pans. You need to get rid of Ca and Cl in a filtration system.
This will be in two steps.
●Drop a resin ball containing HSO3 in your tank. (We’ll ignore the presence of water in the equation). Let’s see what happens. The resin ball will extract calcium from water by swapping it out with the Hydrogen from the SO3.
SO3 has a -1 charge, while Ca has a +2 charge. So, we need two SO3 for each Ca. ->Ca(So3)2
The Ca swaps out for the H atom, freeing it in the water.
Balance out the number of S, O, and H atoms on the left
Ca +2 + 2HSO3 -> Ca(So3)2 + H2(remember hydrogen in its natural state)
We have 1 Ca, 2 H, 2 S, and 6 O atoms on each side!
That takes care of the calcium!
●Now, what about the chlorine in CaCl2? We need to get rid of that element as well.
Let’s drop another resin ball in our pool of water, made up of NaOH. Na has a +1 charge, while OH has a -1 charge (remember that O has a -2 charge while H has a +1 charge).
NaOH+ Cl – -> NaCl + OH –
NaCl should look familiar—it’s table salt, from our first example!
Final step is pretty easy. The excess H+ atoms swapped out from the first resin ball and the OH- molecules from the second resin ball will join together and form water in your pool!
H + + OH – -> H2O.
OR, more correctly:
Our final reaction, putting both resin balls in the same pool:
Now balance out the number of atoms on each side:
CaCl2 + 2HSO3 + 2NaOH -> Ca(SO3)2 + 2NaCl + 2H2O
Compare the numbers to make sure:
1 Ca; 2 Cl, 2 S; 2 Na; 8 O; 4 H -> 1 Ca, 2 S; 2 Na; 2 Cl; 4H; 8 O
Congrats, all the salt from the water are extracted and are stored in the resin balls. In exchange, we created more pure water released into your pool!Bravo!